OpenEDC Versions 1.1 – 1.3
Source data verification (SDV), signatures, reasons for missing values and more audits.

Co-Founder / CEO

Today we are releasing OpenEDC version 1.3, which fulfills all requirements for clinical trials in accordance with Good Clinical Practice (GCP). Below we take a look at all updates since the last post in detail.
Source Data Verification (SDV)
Source Data Verification (SDV) in clinical trials is a critical quality control process in which data is systematically checked to ensure its accuracy, completeness and reliability. This is done by comparing the data documented in the Electronic Data Capture (EDC) system with the original source data as recorded in medical records, laboratory results or other primary documents. For the approval of new drugs and treatments, the SDV process is therefore an indispensable part of quality assurance in clinical trials.
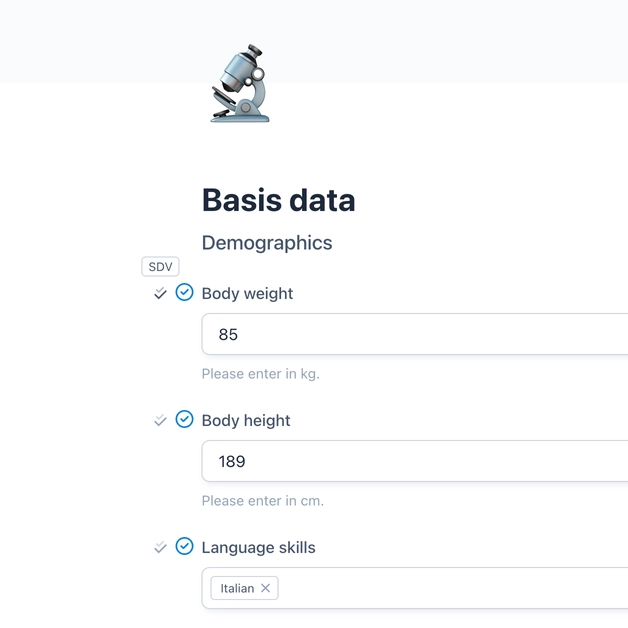
In OpenEDC, Source Data Verification (SDV) can enabled next to each field.
Digital Signatures
In clinical trials, the auditor plays a central role in ensuring data integrity and quality. A digital signature establishes legal responsibility for the correctness of the data collected. It is important that the signatory as well as the date and time of the signature are documented in a clear, attributable and audit-proof manner. To ensure that a signatory is in fact one specific person, a new authentication is required for each signature in OpenEDC.
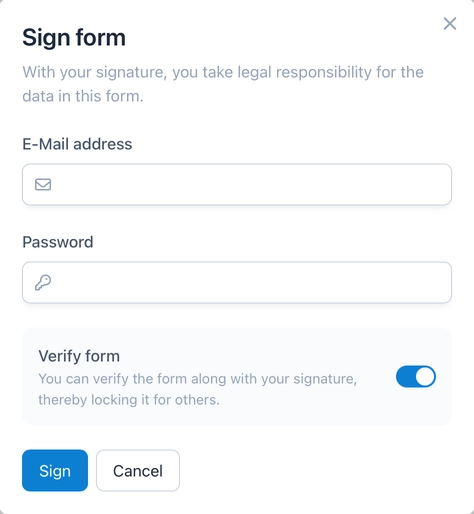
Together with a signature, OpenEDC allows all data in a form to be verified and thus locked for further editing.
Reason for Missing Values
Missing values are a major challenge in research. And data gaps are not uncommon in clinical studies either, be it due to patient dropouts, tests not being carried out or missing answers in questionnaires. However, it is important to document the reason why an entry is missing. This is now possible in OpenEDC and is based on the Null Flavors of HL7 FHIR, which are also used in ISO 21090. In addition to specifying one of the reasons, a comment can be entered to further explain the reason if required.
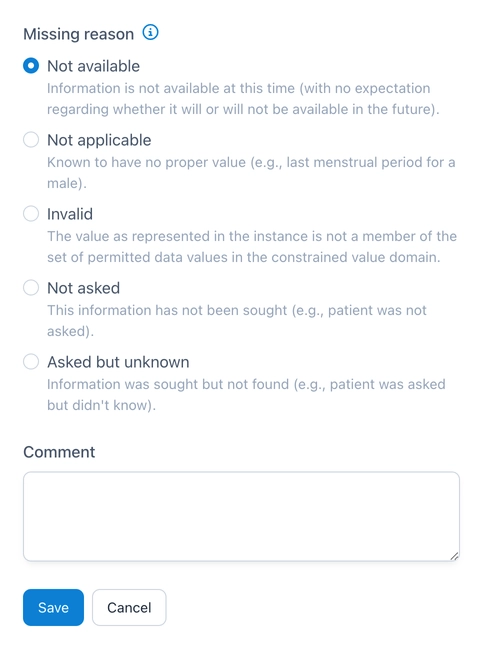
Specifying a reason for a missing value in OpenEDC.
Further Updates
Further changes in the last updates briefly summarized:
Audit trail
The audit trail now also includes changes to the metadata, i.e. the forms. In addition, all changes to all audited data can be viewed in a new tabular overview.
Permissions at form level
Read and write permissions can now be defined at form level. This makes it possible for users to only be able to see or edit certain forms.
Files in reports
Uploaded files can now be viewed and downloaded directly in the report. A preview function is available for PDF, image and video files.
This concludes this article. Thank you for your interest. If you would like to find out more, arrange a free demo or test our app.
