The - simple
- secure
- modern
- simple
solution for clinical trials
A validated system for clinical trials with a focus on user-friendliness, collaboration and data standards. Fully GCP and GDPR compliant.
Request a free demo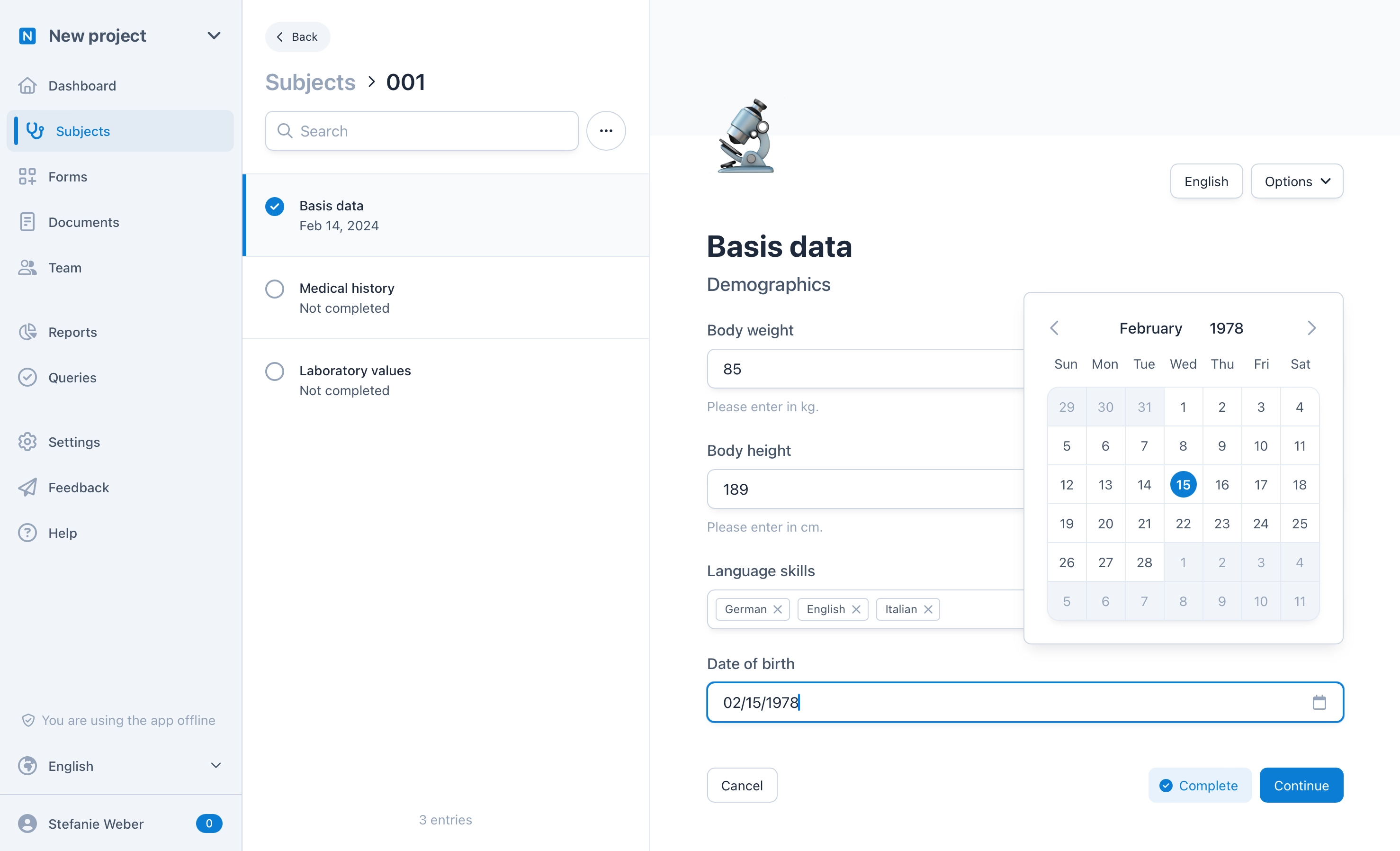

The OpenEDC Cloud is available now!
Developed in Germany, hosted in the Telekom Cloud or On-Premises. Request a free demo.
Application areas
From thesis to clinical trial
OpenEDC is the ideal solution for all types of research projects. From surveys during a doctoral thesis, to clinical outcomes study, to multi-centric studies. The modular concept allows the solution to be customized for every requirement.

Clinical trials
OpenEDC supports clinical research organizations (CROs) as well as medical device and pharmaceutical manufacturers in conducting clinical trials with a validated system. Fully AMG, MPG, DSGVO, GCP and HIPAA compliant.
Outcomes studies
Clinics and practices can use OpenEDC to evaluate and continuously improve the outcome of their therapies. An app for patients allows user-friendly and digital follow-up. Interactive statistics provide insights in real time.
Registers
OpenEDC enables university hospitals, institutes and other research institutions to conduct large longitudinal and multi-centric trials such as registries and epidemiological studies. With the option of distributed data storage.
Doctoral thesis
Students and individual researchers can use OpenEDC locally on their own computers – without installation or registration. Surveys are easy to set up in compliance with the data protection regulations.
Features
A Variety of opportunities
OpenEDC offers you all the essential functions to implement your research project quickly and easily.
Simple form creation
OpenEDC offers an interactive, easy-to-understand form editor. Type in questions and pre-defined answer options in a natural way. See what the finished form looks like at any time. Create multilingual forms with real-time validations, branching and conditional logics, file uploads and much more.
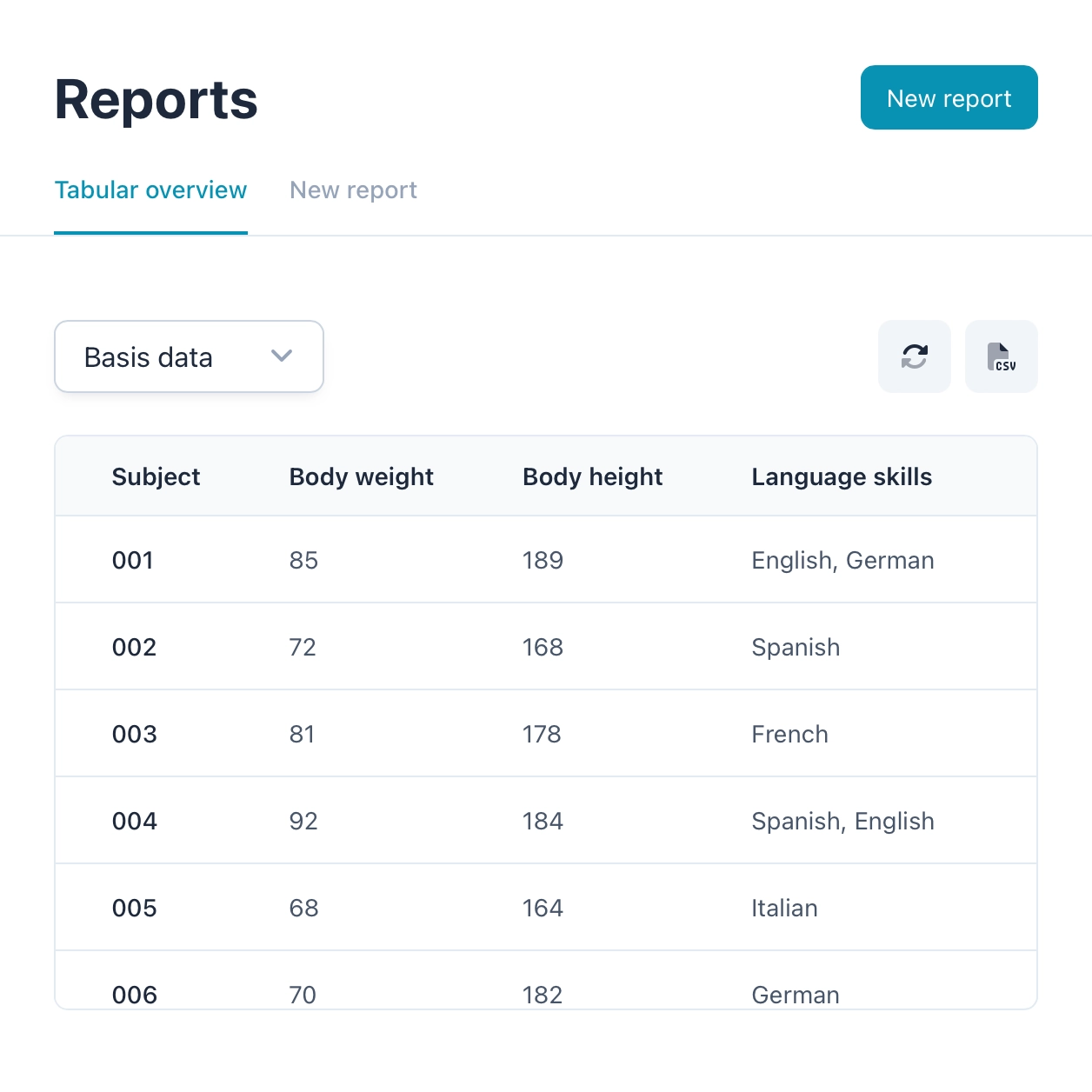
Reports and insights
OpenEDC allows you to create powerful, highly interactive reports. Simply filter data with one click and combine multiple filters until you see the data selection you want. You can then easily export this selection, analyze it further with other statistical tools or share it with colleagues.
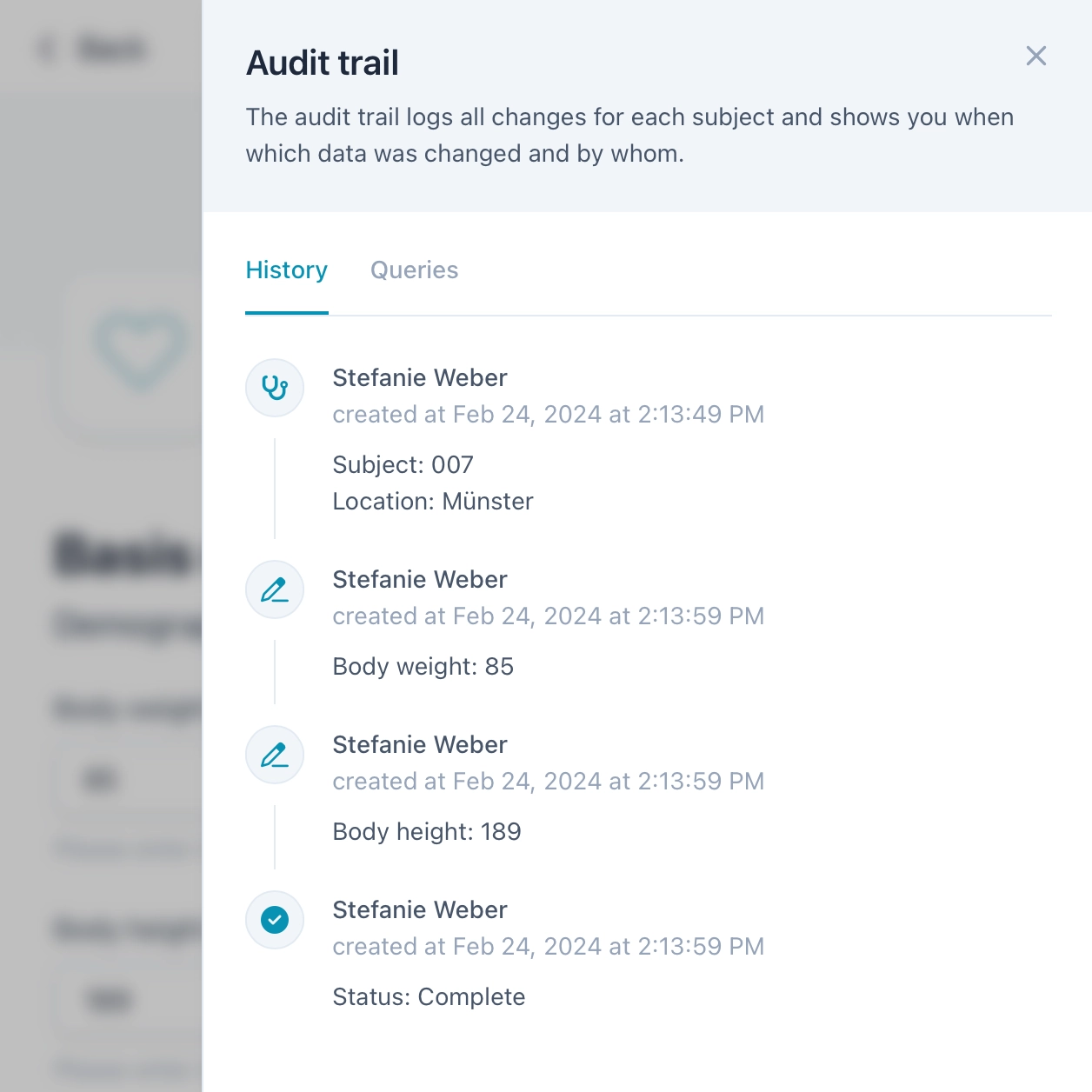
Compliant with GCP
OpenEDC complies with all legal requirements of Good Clinical Practice (GCP). View the audit trail that records all changes in the data set. Create, assign and monitor queries for the highest data quality. And use the granular roles and rights system to define user access permissions.
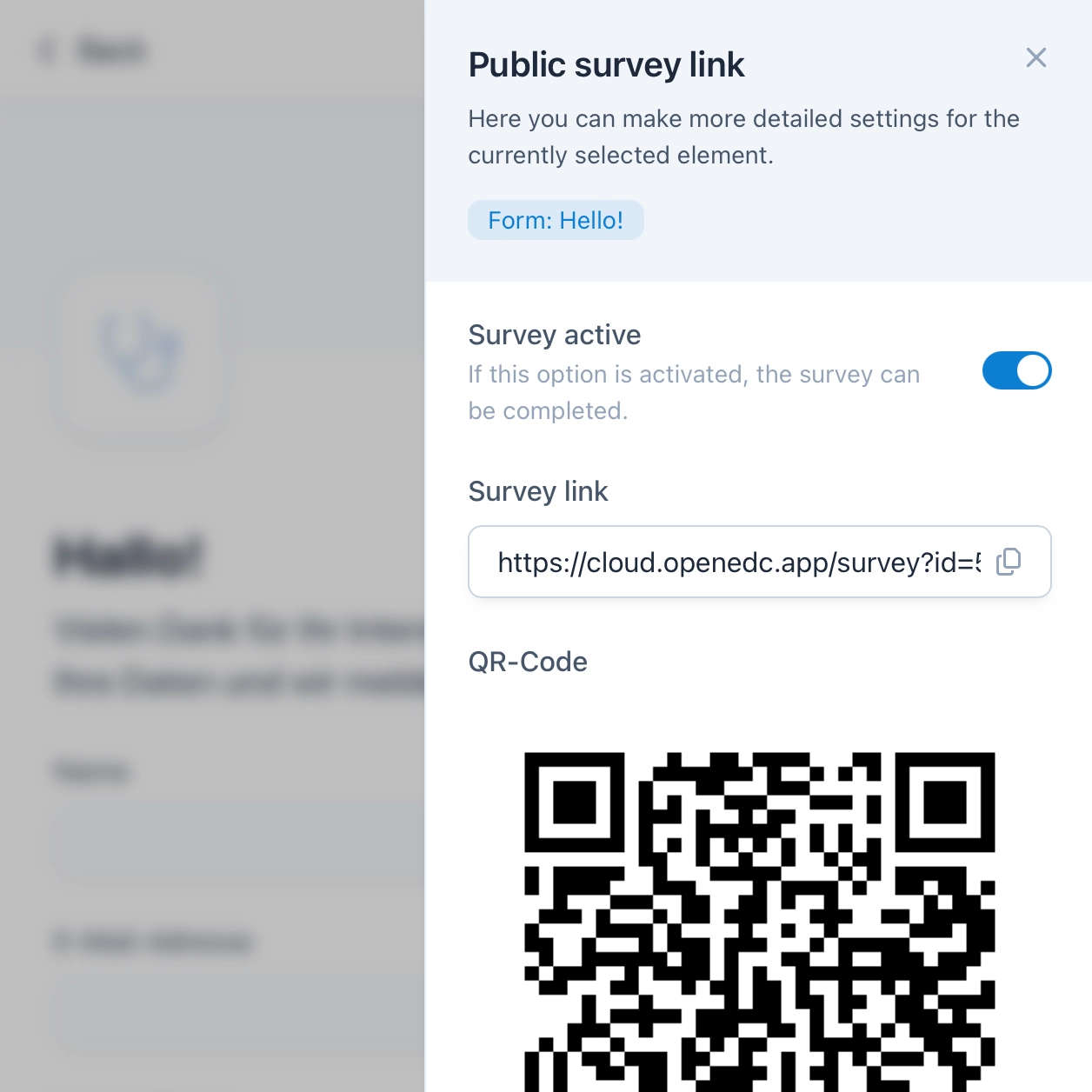
Surveys for ePRO and eCOA
You can send surveys as a link or QR code in a matter of seconds and thus collect electronic patient-reported outcomes (ePROs or eCOA). Additionally, you can give participants the opportunity to register themselves for the study, after which they can use a dedicated participant app for upcoming visits.
Identifiable information. Encrypted.
OpenEDC is the first EDC system that offers an end-to-end encryption for personally identifiable information. For example, you can save the name of a patient in an Informed Consent or eConsent form so that only you and the patient can read it - even we have no technical possibility to decrypt and read this data. This also works biometrically via fingerprint, Face-ID or Windows Hello.
Calender and visits
With the calendar module, everyone in your team can create and manage shared or personal dates to keep everyone up to date. In longitudinal studies, the next visits for each subject are automatically displayed in the calendar - this enables central and efficient organization without any manual scheduling.
More features
OpenEDC offers many features to support you with your study.
-
Pseudonymization
- Manage all participants of your trial – also completely pseudonymized if required.
-
Randomization
- Coming soon: Different methods for the automatic randomization of subjects.
-
Document management
- Revision-proof documents for patient consents, SOPs, wikis or similar.
-
Multi-center studies
- Creation and management of multiple sites for distributed, multi-center studies.
-
Events and visits
- Grouping of related forms for longitudinal studies and research projects.
-
Repeating data
- Patient diaries or medication lists with an arbitrary number of repetitions.
-
Excel und CSV Import
- Existing data can be cleansed, standardized and imported during a guided process.
-
Reason for change
- Specifying a reason for data changes - in accordance with the GCP regulation.

Vision
Our vision
References
Practical experiences
We are advocates for academic research and provide institutes with an customizable and flexible solution for diverse research projects.

“The major advantages of OpenEDC for us are user-friendliness, extensibility and interoperability. By supporting the CDISC ODM data standard, we can export and import both metadata and clinical data in a widely recognized format.”

“Decentralized data storage and record linkage is a vital factor for us. We use it to implement federated projects without disregarding the needs of the participating sites. Each clinic retains control over its data – and yet comprehensive monitoring is possible with OpenEDC.”
Get in contact
Would you like to conduct your study simply, securely and efficiently? Then get in touch and we will be happy to present OpenEDC to you.
